This post is Part 1 on the April 29, 2024 session of the IPAK-EDU Director’s Science Webinar featuring the research and journalism of Robert Whitaker.
Part 2 is HERE.
Antidepressants and SSRIs
Before diving into the STAR*D Scandal, it’s important to consider the context of antidepressant medications from a wider perspective.
For decades, antidepressant medications have resided near the top of global pharmaceuticals market. In 2024 alone, antidepressants are estimated to be a $17 billion industry.1 The familiar key players, in order of market share, are: GlaxoSmithKline, Sanofi, AstraZeneca, Eli Lilly, and Pfizer.2
The most widely prescribed antidepressants belong to a class of drugs known as selective serotonin reuptake inhibitors, or SSRIs. They are commonly used in treatment of depression and anxiety disorders, but also are prescribed for obsessive-compulsive disorder, PTSD, bulimia, and chronic fatigue, among many other conditions.
SSRIs (like vaccines) are touted as ‘safe and effective’.
Eli Lilly began the research developing the ligands3 that were intended to inhibit the natural reuptake of serotonin in the brain. The expected result was that altering the serotonin reuptake cycle would increase serotonin concentrations within the synaptic cleft between neurons (see graphic below), which would thereby increase and stimulate postsynaptic serotonin receptors. By 1974, researchers had reported ‘success’ and suggested that the drug fluoxetine could serve as a treatment for depression. Fluoxetine would go on to be marketed as Prozac.
Zimelidine, developed by the Swedish company Astra AB (predecessor to AstraZeneca), beat Prozac to the punch and was the first SSRI to the European market in 1982, before it was voluntarily withdrawn (just 16 months later) due to reports of Guillain-Barré syndrome attributed to the drug.
Prozac (fluoxetine) eventually was approved by the FDA in 1987 for the US market. In the decades that followed, more SSRIs gained approval, including:
Celexa (citalopram)
Lexapro (escitalopram)
Paxil (paroxetine)
Zoloft (setraline)
Of the 50 top prescribed drugs, 10 are antidepressants, and all 5 of the SSRIs mentioned above are on the list.
Suffice to say, there is a lot of money and interests at stake.
The STAR*D Study
STAR*D = Sequenced Treatment Alternatives to Relieve Depression4
The study, begun in 2001, was the largest and longest study of antidepressants in clinical care ever conducted, ostensibly to ‘evaluate depression treatment’. It was funded to the tune of $35 million by the NIH National Institute of Mental Health [NIMH] as a public health clinical trial. The NIMH is the lead federal agency for research on mental disorders.
Make no mistake: this was a major study run by a Federal agency, and US taxpayers paid the bill.
This is how the NIMH presents STAR*D:
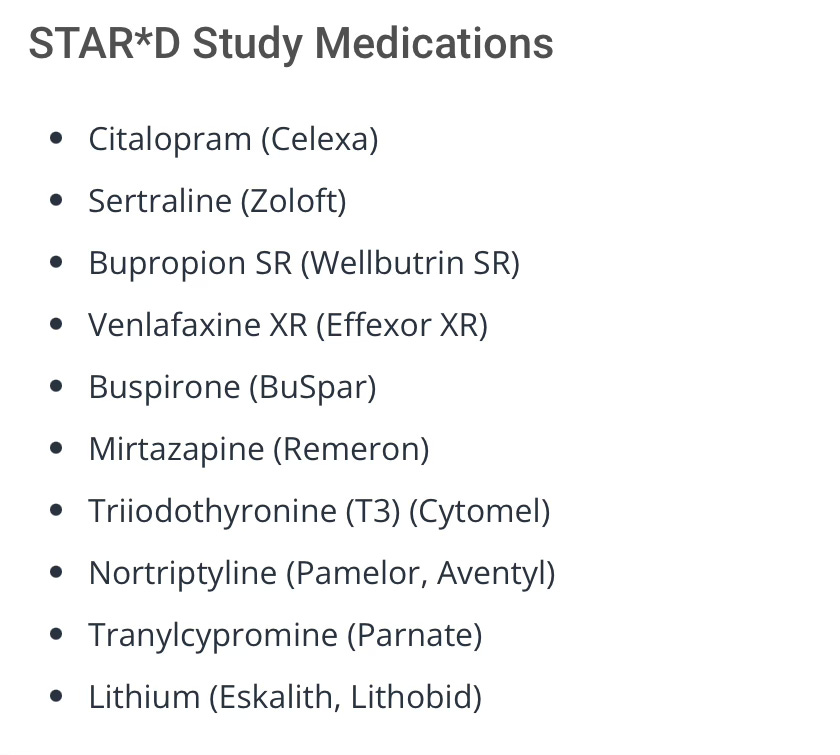
9 antidepressant drugs were ‘donated’ for this study:
The study applied escalating treatment levels to the subjects over a period of seven years, collected patient data, and claimed to report ‘remission’ rates.
In 2006, the NIMH released the initial results. You can read the summary here:
https://www.nimh.nih.gov/funding/clinical-research/practical/stard/allmedicationlevels
The claimed findings were nothing short of incredible (emphasis added):
In conclusion, about half of participants in the STAR*D study became symptom-free after two treatment levels. Over the course of all four treatment levels, almost 70 percent of those who did not withdraw from the study became symptom-free.
Since results were published, STAR*D has generated an expansive constellation of related articles and has been widely referenced, with thousands of citations, according to Google Scholar.
As an example, an article by members of the STAR*D author team, in the journal Psychiatric Services, claimed a 67% cumulative remission rate. This hugely positive statistic is the message that media outlets like The New Yorker would pick up and run with.
But when the STAR*D study was deconstructed and the data re-analyzed—using the study’s own protocols—a shocking discovery was made.
Enter: Ed Pigott
Deconstructing STAR*D
When results were published by NIMH in 2006, psychologist Edmund Pigott grew concerned with apparent author biases in the study, and began to dig deep. He devoted 5 years deconstructing STAR*D. That effort was solidified and published in the journal Psychotherapy and Psychosomatics in 2010. The article was entitled, “Efficacy and Effectiveness of Antidepressants: Current Status of Research”.5
“Before STAR*D, I wasn’t prone to believing in conspiracy theories but as we peeled back its layers I found myself spinning theories about how this study became so profoundly misrepresented given all of the actors involved (20+ top-tier researchers/NIMH), any one of whom could have objected and ‘blown the whistle;’ but none did.”
- Ed Pigott
He then began blogging on his findings at Robert Whitaker’s Mad In America in 2011.
Pigott, working with a team, would go on to get a reanalysis of the STAR*D data published in the BMJ in 2023.6 The findings were damning. Take a look at the Results and Conclusion captured in the screenshot below for a glimpse of what was uncovered.
It appeared that in determining the remission rate trumpeted by NIMH, the authors had deviated from the study’s own protocol. The NIMH claimed remission rate was greatly exaggerated, inflated by nearly a factor of 2. Had the protocols been adhered to, the remission rate would have been 35%.
Perhaps even more significantly, the STAR*D investigators had failed to report the stay-well rate after a year. Analyzing the data, Pigott found that just 3% of the patients who started the trial stayed well at the end of the study. Does it matter that some sort of remission was recorded if it was temporary, fleeting?
Enter: Robert Whitaker
Robert is a journalist with principles, an all too rare thing these days, regrettably.
In the introduction to his talk, Robert spoke to what drove him to look at the STAR*D scandal and his view on the essential responsibility journalists have in relating science to the public.
Meet Robert Whitaker.
Robert would go on to give voice to Pigott’s findings and pen a series of pieces on the STAR*D Scandal and what he called “Scientific Misconduct on a Grand Scale.”
In the clips that follow, Whitaker outlines STAR*D and the evidence of fraud. He begins by emphasizing the importance of the study: it is the benchmark study that has governed the use of antidepressants in clinical care since 2006.
This bears repeating and underscoring: STAR*D is likely, the most influential benchmark study on antidepressants, and it is neck-deep in fraud.
Watch.
To be continued in Part 2.
Stay tuned.
Follow Robert Whitaker at Mad In America.
#twistingstrands
#questioneverything
Information wants to be free—and over 90% of the content here is accessible to anyone. But everything takes care and time, and your support matters. If you like what you see, and you’re willing and able, consider leaving a tip. Every little bit helps. Thank you!
Paid subscribers to Twisting Strands will get access to a growing catalog of video shorts for an easy-to-watch, deeper perspective. Consider upgrading your subscription—you’ll be glad you did.
Subscribers to the IPAK-EDU Director’s Science Webinar get full access to the catalog of webinar recordings, including this session with Robert Whitaker.
Your support of the webinar and IPAK-EDU makes this possible!
https://www.snsinsider.com/reports/antidepressants-market-3074
https://www.mordorintelligence.com/industry-reports/antidepressants-market/market-share
A ligand is the signaling molecule which can reversibly bind to the receiving molecule, or receptor. The word stems from the Latin ligare ‘to bind’ and its predecessor Proto-Indo-European root leig- ‘to tie’.
https://www.nimh.nih.gov/funding/clinical-research/practical/stard
Just a starting point, there are many STAR*D related papers:
https://doi.org/10.1016/S0197-2456(03)00112-0
H. Edmund Pigott, Allan M. Leventhal, Gregory S. Alter, John J. Boren; Efficacy and Effectiveness of Antidepressants: Current Status of Research. Psychother Psychosom 1 August 2010; 79 (5): 267–279. https://doi.org/10.1159/000318293
Pigott HE, Kim T, Xu C, et. al. What are the treatment remission, response and extent of improvement rates after up to four trials of antidepressant therapies in real-world depressed patients? A reanalysis of the STAR*D study’s patient-level data with fidelity to the original research protocol. BMJ Open 2023;13:e063095. https://doi.org/10.1136/bmjopen-2022-063095